Abstract
Background: Multiple myeloma (MM) is a disease of older adults with a median age of onset of 70 years. Approximately 20% of the incident cases are diagnosed in patients 85 years and older. There is significant heterogeneity among older adults with regards to the tolerability of myeloma directed therapy. In newly diagnosed transplant ineligible patients with MM, available evidence indicates that age greater than 80 is an independent risk factor for adverse clinical outcomes ("frail"). However, there are limited data on outcomes in adults 80 years and older in the relapsed or refractory disease setting. Over the past few years, the therapy armamentarium for patients with relapsed or refractory MM has expanded considerably with approval of at least 5 new drugs including two monoclonal antibodies, an HDAC inhibitor, and an oral proteasome inhibitor. The impact of these recent drug approvals on clinical outcomes in adults 80 years and older with relapsed or refractory MM is also unknown. This pooled analysis attempts to evaluate outcomes by age in patients with relapsed or refractory multiple myeloma
Methods: Data from 10 clinical trials submitted to support approval of drugs for patients with relapsed or refractory multiple myeloma from 2011-2018 were pooled for this analysis. Participants were grouped according to three age strata: <75, 75-80 and >80 years. Clinical outcomes including response rates (ORR, CR, and PR) in the different age groups were analyzed. Preliminary results of response rates by age are presented.
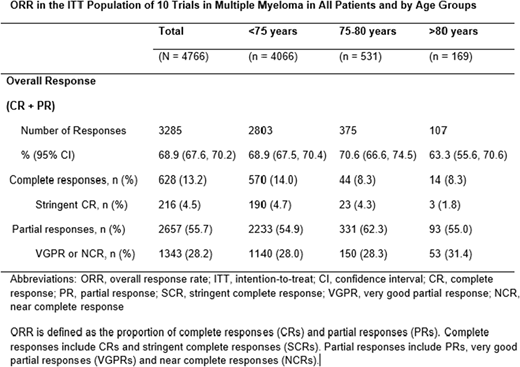
Results: We identified 4766 patients enrolled in 10 clinical trials of relapsed or refractory multiple myeloma. The median age of the patient population was 64.5 (range 30.0-91.0). Eighty-five percent of the patients were less than 75 years of age, 11% were between 75-80 years of age, and only 4% were older than 80 years of age. Seventy percent of the population was classified as ISS Stage I or II and 24% was ISS Stage III. Overall response rate (ORR) in the pooled studies was 69% (67.6, 70.2) with less than 15% of the responses being complete responses (CR). ORR was 63.3 % (55.6, 70.6) in those 80 years and older; 70.6 % (66.6, 74.5) in the 75-80 years age group; and 68.9 % (67.5, 70.4) in patients younger than 75 years of age. CR rates were 8.3% in both the 75-80 age group and those older than 80 years, and 14.0% in those younger than 75 years.
Conclusion: In this large database of recent approvals for the treatment of RRMM, preliminary results show no significant differences in ORR between patients over 80 years and those 80 years and younger (difference -5.4%; 95% CI (-13.1%, 2.3%)) suggesting response to treatment is independent of age. However, our results need to be interpreted with caution considering differences in patient populations particularly with respect to different treatment regimens and therapies. Analysis of survival outcomes is ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal